
|
Lobby > Exhibits > Why Geysers Erupt > Geyser Ingredients > Heat HeatA BRIEF INTRODUCTION
Earth's heat is released by both
conduction and convection in Yellowstone. Conduction
occurs when heat is transferred from something hot to something
colder.
If you stick one end of a metal rod into a fire, the energy from
the heat will excite or agitate the molecules in the metal. Those
excited molecules will collide with nearby cooler ones, and transfer
energy to them, making them hotter. Eventually, if you hold onto
the rod long enough, the heat will be transferred to your hand.
That's conduction (and a possible burn, if you don't let go of the
rod). So much for dry explantions; let's move on to "cooler" stuff! Next Section: Earth's Oven |
|
||||||||||||||||||||||||||||||||
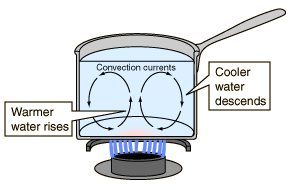 Convection occurs when heat is transferred by the movement of hot liquids or gases, such as air, water, or magma, to a cooler area. We've all learned that hot air rises.
This occurs because air expands as it heats, becoming less dense and more buoyant than the surrounding cooler air. The farther the air gets from its heat source, the cooler and denser it becomes, until eventually it begins to sink again. Baseboard heating in a house, hot air balloons, and water boiling in a pot are all examples of convection.
Convection occurs when heat is transferred by the movement of hot liquids or gases, such as air, water, or magma, to a cooler area. We've all learned that hot air rises.
This occurs because air expands as it heats, becoming less dense and more buoyant than the surrounding cooler air. The farther the air gets from its heat source, the cooler and denser it becomes, until eventually it begins to sink again. Baseboard heating in a house, hot air balloons, and water boiling in a pot are all examples of convection.
